What Is The Chemical Makeup Of Doxycycline Hyclate
 | |
 | |
| Clinical information | |
|---|---|
| Pronunciation | DOKS-iss-EYE-kleen |
| Trade names | Doxy, Doryx, Vibramycin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682063 |
| License data |
|
| Pregnancy category |
|
| Routes of assistants | Past mouth, intravenous[i] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | fourscore–90% |
| Metabolism | Negligible |
| Emptying half-life | 10–22 hours |
| Excretion | Mainly faeces, twoscore% urine |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.008.429 |
| Chemical and physical data | |
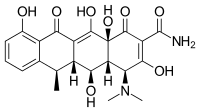
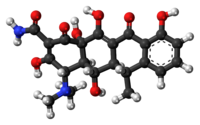
| Formula | C 22 H 24 N 2 O 8 |
| Molar mass | 444.440 m·mol−1 |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
| | |
Doxycycline is a broad-spectrum tetracycline-form antibody used in the treatment of infections acquired past leaner and certain parasites.[1] It is used to care for bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, and syphilis.[1] It is besides used to foreclose malaria in combination with quinine.[1] Doxycycline may be taken by mouth or past injection into a vein.[i]
Mutual side effects include diarrhea, nausea, airsickness, and an increased risk of sunburn.[1] Use during pregnancy is not recommended.[one] Doxycycline is a broad-spectrum antibiotic, of the tetracycline class.[1] Similar other agents of the tetracycline course, it either slows or kills bacteria by inhibiting protein production.[ane] [2] It kills malaria by targeting a plastid organelle, the apicoplast.[3] [four]
Doxycycline was patented in 1957 and came into commercial use in 1967.[5] [6] It is on the World Health Arrangement's List of Essential Medicines.[7] Doxycycline is available as a generic medicine.[1] [8] In 2019, it was the 90th near ordinarily prescribed medication in the Usa, with more than eightmillion prescriptions.[ix] [x]
Medical use [edit]
Generic 100 mg doxycycline capsules

In improver to the general indications for all members of the tetracycline antibiotics group, doxycycline is ofttimes used to care for Lyme disease, chronic prostatitis, sinusitis, pelvic inflammatory illness,[11] [12] acne, rosacea,[13] [14] and rickettsial infections.[xv]
In Canada, in 2004, doxycycline was considered a commencement-line treatment for chlamydia and non-gonococcal urethritis and with cefixime for uncomplicated gonorrhea.[16]
Antibacterial [edit]
Moraxella catarrhalis, Brucella melitensis, Chlamydia pneumoniae, and Mycoplasma pneumoniae are generally susceptible to doxycycline, while some Haemophilus spp., Mycoplasma hominis, and Pseudomonas aeruginosa accept developed resistance to varying degrees.[17]
It is used in the handling and prophylaxis of anthrax and Leptospirosis.[18] It is also effective against Yersinia pestis (the infectious agent of bubonic plague), and is prescribed for the treatment of Lyme illness,[19] [20] [21] [22] ehrlichiosis,[23] [24] and Rocky Mountain spotted fever.[25]
Doxycycline is indicated for treatment of:[25] [26]
- Rocky Mountain spotted fever, typhus fever and the typhus grouping, Q fever,[27] rickettsialpox, and tick fevers acquired past Rickettsia
- Respiratory tract infections caused by Mycoplasma pneumoniae [28]
- Lymphogranuloma venereum, trachoma, inclusion conjunctivitis, and uncomplicated urethral, endocervical, or rectal infections in adults acquired past Chlamydia trachomatis
- Psittacosis
- Nongonococcal urethritis caused by Ureaplasma urealyticum
- Relapsing fever due to Borrelia recurrentis
- Chancroid caused past Haemophilus ducreyi
- Plague due to Yersinia pestis
- Tularemia
- Cholera
- Campylobacter fetus infections
- Brucellosis caused by Brucella species (in conjunction with streptomycin)
- Bartonellosis
- Granuloma inguinale (Klebsiella species)
- Lyme disease[29]
When bacteriologic testing indicates appropriate susceptibility to the drug, doxycycline may be used to care for these infections caused past Gram-negative bacteria:[25] [26]
- Escherichia coli infections
- Enterobacter aerogenes (formerly Aerobacter aerogenes) infections
- Shigella species infections
- Acinetobacter species (formerly Mima species and Herellea species) infections
- Respiratory tract infections acquired past Haemophilus influenzae
- Respiratory tract and urinary tract infections caused by Klebsiella species
Some Gram-positive bacteria have developed resistance to doxycycline. Upwards to 44% of Streptococcus pyogenes and upwardly to 74% of Due south. faecalis specimens take developed resistance to the tetracycline group of antibiotics. Upwards to 57% of P. acnes strains developed resistance to doxycycline.[thirty] When bacteriologic testing indicates appropriate susceptibility to the drug, doxycycline may be used to treat these infections caused by Gram-positive leaner:[25] [26]
- Upper respiratory infections acquired by Streptococcus pneumoniae (formerly Diplococcus pneumoniae)
- Skin and soft tissue infections caused by Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus infections
- Anthrax caused by Bacillus anthracis infection
When penicillin is contraindicated, doxycycline can be used to treat:[25] [26]
- Syphilis caused past Treponema pallidum
- Yaws caused by Treponema pertenue
- Listeriosis due to Listeria monocytogenes
- Vincent'southward infection caused by Fusobacterium fusiforme
- Actinomycosis caused past Actinomyces israelii
- Infections acquired by Clostridium species
Doxycycline may also exist used as adjunctive therapy for severe acne.[25] [26]
The beginning-line handling for brucellosis is a combination of doxycycline and streptomycin and the 2nd-line is a combination of doxycycline and rifampicin (rifampin).[31]
Antimalarial [edit]
Doxycycline is active confronting the erythrocytic stages of Plasmodium falciparum but not against the gametocytes of P. falciparum.[32] Information technology is used to prevent malaria.[33] It is not recommended lonely for initial treatment of malaria, even when the parasite is doxycycline-sensitive, because the antimalarial event of doxycycline is delayed.[34]
The World Health Arrangement (WHO) guidelines country that the combination of doxycycline with either artesunate or quinine may be used for the treatment of unproblematic malaria due to P. falciparum or following intravenous treatment of severe malaria.[35]
Antihelminthic [edit]
Doxycycline kills the symbiotic Wolbachia bacteria in the reproductive tracts of parasitic filarial nematodes, making the nematodes sterile, and thus reducing transmission of diseases such as onchocerciasis and elephantiasis.[36] Field trials in 2005 showed an eight-week grade of doxycycline almost completely eliminates the release of microfilariae.[37]
Spectrum of susceptibility [edit]
Doxycycline has been used successfully to care for sexually transmitted, respiratory, and ophthalmic infections. Representative pathogenic genera include Chlamydia, Streptococcus, Ureaplasma, Mycoplasma, and others. The following represents MIC susceptibility data for a few medically significant microorganisms.[38]
- Chlamydia psittaci: 0.03 μg/mL
- Mycoplasma pneumoniae: 0.016 μgrand/mL — 2 μgrand/mL
- Streptococcus pneumoniae: 0.06 μg/mL — 32 μg/mL
Sclerotherapy [edit]
Doxycycline is also used for sclerotherapy in deadening-menstruum vascular malformations, namely venous and lymphatic malformations, too every bit post-operative lymphoceles.[39]
Others [edit]
Subantimicrobial-dose doxycycline (SDD) is widely used as an adjunctive handling to scaling and root planing for periodontitis. Significant differences were observed for all investigated clinical parameters of periodontitis in favor of the scaling and root planing + SDD grouping where SDD dosage regimens is 20 mg twice daily for 3 months in a meta-assay published in 2011.[forty]
Contraindications [edit]
Pregnancy and lactation [edit]
Doxycycline is categorized past the FDA every bit a class D drug in pregnancy. Doxycycline crosses into breastmilk.[41] Other tetracycline antibiotics are contraindicated in pregnancy and upwardly to viii years of age, due to the potential for disrupting bone and tooth evolution.[42] They include a class alarm almost staining of teeth and decreased evolution of dental enamel in children exposed to tetracyclines in utero, during breastfeeding or during young childhood.[43] However, the FDA has acknowledged that the actual adventure of dental staining of chief teeth is undetermined for doxycycline specifically. The best available evidence indicates that doxycycline has trivial or no effect on hypoplasia of dental enamel or on staining of teeth and the CDC recommends the use of doxycycline for treatment of Q fever and besides for tick-borne rickettsial diseases in young children and others abet for its use in malaria.[44]
Other [edit]
Other contraindications are severe liver affliction and concomitant apply of isotretinoin or other retinoids, as both tetracyclines and retinoids tin can cause intracranial hypertension (increased pressure around the brain) in rare cases.[45]
Adverse effects [edit]
Adverse effects are similar to those of other members of the tetracycline antibiotic grouping. Doxycycline can cause gastrointestinal upset.[46] [47] Oral doxycycline can cause pill esophagitis, particularly when it is swallowed without adequate fluid, or by persons with difficulty swallowing or impaired mobility.[48] Doxycycline is less likely than other antibiotic drugs to crusade Clostridium difficile colitis.[49]
An erythematous rash in sun-exposed parts of the body has been reported to occur in 7.3–21.ii% of persons taking doxycycline for malaria prophylaxis. One written report examined the tolerability of diverse malaria prophylactic regimens and establish doxycycline did not cause a significantly higher percent of all skin events (photosensitivity not specified) when compared with other antimalarials. The rash resolves upon discontinuation of the drug.[50]
Different another members of the tetracycline group, it may be used in those with renal impairment.[51]
Doxycycline use has been associated with increased take chances of inflammatory bowel disease.[52] In i large retrospective written report, patients who were prescribed doxycycline for their acne had a 2.25-fold greater risk of developing Crohn's disease.[53]
Interactions [edit]
The combination of doxycycline with dairy, antacids, calcium supplements, iron products, laxatives containing magnesium, or bile acid sequestrants is not inherently dangerous, but any of these foods and supplements may subtract doxycycline'southward effectiveness.[45] [54]
Breakfast was observed to reduce doxycycline assimilation significantly. Assimilation of tetracycline occurs in the stomach and the upper modest intestine. Assimilation of tetracyclines has been reported to be impaired by milk products, aluminum hydroxide gels, sodium bicarbonate, calcium and magnesium salts, laxatives containing magnesium and iron preparations. The mechanisms responsible for decreased assimilation appear to be chelation and an increment in gastric pH. ... In view of these results, it is advisable to instruct the patients to take doxycycline on an empty stomach.[55]
Previously, doxycycline was believed to impair the effectiveness of many types of hormonal contraception due to CYP450 induction. Enquiry has shown no pregnant loss of effectiveness in oral contraceptives while using most tetracycline antibiotics (including doxycycline), although many physicians notwithstanding recommend the use of barrier contraception for people taking the drug to foreclose unwanted pregnancy.[56] [57] [58]
Pharmacology [edit]
Doxycycline, similar other tetracycline antibiotics, is bacteriostatic. Information technology works past preventing bacteria from reproducing through the inhibition of poly peptide synthesis.[59]
Doxycycline is highly lipophilic so tin easily enter cells, pregnant the drug is easily absorbed afterward oral administration and has a large volume of distribution. Information technology can likewise exist re-absorbed in the renal tubules and gastrointestinal tract due to its high lipophillicity then has a long elimination half life, and does not accumulate in the kidneys of patients with kidney failure due to the compensatory excretion in faeces.[47] [60] Doxycycline–metal ion complexes are unstable at acid pH, therefore more doxycycline enters the duodenum for absorption than the earlier tetracycline compounds. In addition, nutrient has less effect on absorption than on assimilation of earlier drugs with doxycycline serum concentrations beingness reduced by about 20% by exam meals compared with l% for tetracycline.[61]
Mechanism of activity [edit]
Doxycycline is a broad-spectrum antibiotic. It inhibits the synthesis of bacterial proteins by binding to the 30S ribosomal subunit, which is only establish in bacteria.[46] [sixty] This prevents the binding of transfer RNA to messenger RNA at the ribosomal subunit significant amino acids cannot be added to polypeptide chains and new proteins cannot be made. This stops bacterial growth giving the immune organization time to kill and remove the leaner.[62]
Pharmacokinetics [edit]
The substance is about completely absorbed from the upper part of the pocket-size intestine. It reaches highest concentrations in the claret plasma after 1 to two hours and has a high plasma protein binding rate of about lxxx–xc%. Doxycycline penetrates into about all tissues and body fluids. Very loftier concentrations are constitute in the gallbladder, liver, kidneys, lung, breast milk, bone and genitals; low ones in saliva, aqueous sense of humor, cerebrospinal fluid (CSF), and especially in inflamed meninges.[45] [63] [64] Past comparing, the tetracycline antibody minocycline penetrates significantly better into the CSF and meninges.[65]
Doxycycline metabolism is negligible. Information technology is actively excreted into the gut (in part via the gallbladder, in part directly from blood vessels), where some of information technology is inactivated by forming chelates. Virtually 40% are eliminated via the kidneys, much less in people with end-stage kidney affliction. The biological half-life is 18 to 22 hours (16±six hours co-ordinate to some other source[63]) in healthy people, slightly longer in those with end-stage kidney disease, and significantly longer in those with liver disease.[45] [63] [64]
Chemistry [edit]
Expired tetracyclines or tetracyclines allowed to stand at a pH less than ii are reported to be nephrotoxic due to the germination of a degradation product, anhydro-four-epitetracycline[66] [67] causing Fanconi syndrome.[68] In the case of doxycycline, the absence of a hydroxyl group in C-6 prevents the formation of the nephrotoxic compound.[67] Nonetheless, tetracyclines and doxycycline itself have to be taken with circumspection in patients with kidney injury, every bit they tin can worsen azotemia due to catabolic effects.[68]
Chemic properties [edit]
Doxycycline, doxycycline monohydrate and doxycycline hyclate are xanthous, crystalline powders with a bitter taste. The latter smells faintly of ethanol; a ane% aqueous solution has a pH of two–3; and the specific rotation is −110° cm³/dm·g in 0.01 N methanolic hydrochloric acid.[63]
| Solubility in | Doxycycline | Doxycycline monohydrate | Doxycycline Hyclate |
|---|---|---|---|
| H2o | very slightly | very slightly | freely |
| Ethanol | very slightly | very slightly | sparingly |
| Aqueous acids | freely | freely | |
| Alkali hydroxyde solutions | freely | freely | |
| Chloroform | very slightly | practically insoluble | practically insoluble |
| Diethyl ether | insoluble | practically insoluble | practically insoluble |
History [edit]
After penicillin revolutionized the handling of bacterial infections in WWII, many chemic companies moved into the field of discovering antibiotics past bioprospecting. American Cyanamid was one of these, and in the late 1940s chemists at that place discovered chlortetracycline, the starting time member of the tetracycline grade of antibiotics.[2] Shortly thereafter, scientists at Pfizer discovered terramycin and it was brought to marketplace. Both compounds, like penicillin, were natural products and information technology was ordinarily believed that nature had perfected them, and further chemical changes could only degrade their effectiveness. Scientists at Pfizer led by Lloyd Conover modified these compounds, which led to the invention of tetracycline itself, the start semi-synthetic antibiotic. Charlie Stephens' group at Pfizer worked on further analogs and created one with greatly improved stability and pharmacological efficacy: doxycycline. It was clinically developed in the early 1960s and approved by the FDA in 1967.[2]
As its patent grew most to expiring in the early on 1970s, the patent became the subject of lawsuit between Pfizer and International Rectifier[69] that was not resolved until 1983; at the fourth dimension it was the largest litigated patent case in The states history.[70] Instead of a cash payment for infringement, Pfizer took the veterinary and feed-additive businesses of International Rectifier'south subsidiary, Rachelle Laboratories.[70]
In Jan 2013, the FDA reported shortages of some, but not all, forms of doxycycline "acquired by increased need and manufacturing issues".[71] Companies involved included an unnamed major generics manufacturer that ceased production in February 2013, Teva (which ceased production in May 2013), Mylan, Actavis, and Hikma Pharmaceuticals.[72] [73] The shortage came at a specially bad time, since at that place were also shortages of an culling antibiotic, tetracycline, at the same time.[74] The market cost for doxycycline dramatically increased in the United States in 2013 and early on 2014 (from $xx to over $1800 for a canteen of 500 tablets),[75] [76] [77] earlier decreasing over again.[78] [79]
Order and culture [edit]
Doxycycline is available worldwide under many brand names.[80] Doxycycline is available equally a generic medicine.[1] [8]
Enquiry [edit]
Research areas have included:
- Macular degeneration[81]
- Rheumatoid arthritis instead of minocycline (both of which have demonstrated modest efficacy for this illness)[82]

Tet-ON inducible shRNA arrangement
Enquiry reagent [edit]
Doxycycline and other members of the tetracycline course of antibiotics are often used equally inquiry reagents in in vitro and in vivo biomedical research experiments involving bacteria besides in experiments in eukaryotic cells and organisms with inducible protein expression systems using tetracycline-controlled transcriptional activation. The mechanism of action for the antibacterial event of tetracyclines relies on disrupting protein translation in bacteria, thereby damaging the ability of microbes to grow and repair; however poly peptide translation is also disrupted in eukaryotic mitochondria impairing metabolism and leading to furnishings that tin can confound experimental results.[83] [84] Doxycycline is also used in "tet-on" (factor expression activated past doxycycline) and "tet-off" (cistron expression inactivated past doxycycline) tetracycline-controlled transcriptional activation to regulate transgene expression in organisms and cell cultures.[85] Doxycycline is more stable than tetracycline for this purpose.[85] At subantimicrobial doses, doxycycline is an inhibitor of matrix metalloproteases, and has been used in various experimental systems for this purpose, such as for recalcitrant recurrent corneal erosions.[86]
References [edit]
- ^ a b c d due east f g h i j thou "Doxycycline calcium". The American Social club of Health-Arrangement Pharmacists. Archived from the original on 23 September 2015. Retrieved 18 Baronial 2015.
- ^ a b c Nelson ML, Levy SB (December 2011). "The history of the tetracyclines". Register of the New York Academy of Sciences. 1241 (1): 17–32. Bibcode:2011NYASA1241...17N. doi:10.1111/j.1749-6632.2011.06354.x. PMID 22191524. S2CID 34647314.
- ^ McFadden GI (March 2014). "Apicoplast". Current Biology. 24 (7): R262-iii. doi:10.1016/j.cub.2014.01.024. PMID 24698369.
- ^ Schlagenhauf-Lawlor, Patricia (2008). Travelers' Malaria. PMPH-U.s.a.. p. 148. ISBN9781550093360.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Counterpart-based Drug Discovery. John Wiley & Sons. p. 489. ISBN9783527607495.
- ^ Corey, E.J. (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 406. ISBN9781118354469.
- ^ World Health Organization (2019). World Wellness System model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC By-NC-SA 3.0 IGO.
- ^ a b Hamilton, Richard J. (2011). Tarascon pharmacopoeia (12th ed.). Sudbury, MA: Jones & Bartlett Learning. p. 79. ISBN9781449600679.
- ^ "The Top 300 of 2019". ClinCalc . Retrieved xvi October 2021.
- ^ "Doxycycline - Drug Usage Statistics". ClinCalc . Retrieved sixteen October 2021.
- ^ Sweet RL, Schachter J, Landers DV, Ohm-Smith M, Robbie MO (March 1988). "Handling of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline". American Journal of Obstetrics and Gynecology. 158 (3 Pt 2): 736–41. doi:10.1016/S0002-9378(xvi)44537-0. PMID 3162653.
- ^ Gjønnaess H, Holten E (1978). "Doxycycline (Vibramycin) in pelvic inflammatory affliction". Acta Obstetricia et Gynecologica Scandinavica. 57 (ii): 137–9. doi:10.3109/00016347809155893. PMID 345730. S2CID 28328073.
- ^ Määttä K, Kari O, Tervahartiala T, Peltonen S, Kari K, Saari M, Sorsa T (August 2006). "Tear fluid levels of MMP-8 are elevated in ocular rosacea--treatment outcome of oral doxycycline". Graefe'southward Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie. 244 (8): 957–62. doi:10.1007/s00417-005-0212-3. PMID 16411105. S2CID 20540747.
- ^ Quarterman MJ, Johnson DW, Abele DC, Lesher JL, Hull DS, Davis LS (January 1997). "Ocular rosacea. Signs, symptoms, and tear studies earlier and after treatment with doxycycline". Archives of Dermatology. 133 (1): 49–54. doi:10.1001/archderm.133.1.49. PMID 9006372.
- ^ Walker DH, Paddock CD, Dumler JS (November 2008). "Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections". The Medical Clinics of North America. 92 (6): 1345–61, x. doi:10.1016/j.mcna.2008.06.002. PMID 19061755.
- ^ Michael L. Rekart (December 2014). "Doxycycline: "New" treatment of choice for genital chlamydia infections". Archived from the original on 2 February 2017.
- ^ "Doxycycline spectrum of bacterial susceptibility and Resistance" (PDF). Archived from the original (PDF) on 1 February 2014. Retrieved 4 May 2012.
- ^ Stoddard, Robyn A.; Galloway, Renee 50.; Guerra, Marta A. (10 July 2015). "Leptospirosis - Chapter 3". wwwnc.cdc.gov. Atlanta, GA: Centers for Disease Command and Prevention. Archived from the original on 9 Apr 2017. Retrieved sixteen Apr 2017.
- ^ Nadelman RB, Luger SW, Frank Eastward, Wisniewski Thou, Collins JJ, Wormser GP (August 1992). "Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme affliction". Annals of Internal Medicine. 117 (four): 273–80. doi:10.7326/0003-4819-117-4-273. PMID 1637021.
- ^ Luger SW, Paparone P, Wormser GP, Nadelman RB, Grunwaldt E, Gomez G, et al. (March 1995). "Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans". Antimicrobial Agents and Chemotherapy. 39 (three): 661–7. doi:10.1128/AAC.39.three.661. PMC162601. PMID 7793869.
- ^ Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman Thousand, McKenna D, et al. (July 2001). "Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite". The New England Journal of Medicine. 345 (2): 79–84. doi:x.1056/NEJM200107123450201. PMID 11450675.
- ^ Karlsson M, Hammers-Berggren S, Lindquist L, Stiernstedt Yard, Svenungsson B (July 1994). "Comparison of intravenous penicillin G and oral doxycycline for handling of Lyme neuroborreliosis". Neurology. 44 (7): 1203–7. doi:10.1212/WNL.44.7.1203. PMID 8035916. S2CID 38661885.
- ^ Weinstein RS (November 1996). "Human ehrlichiosis". American Family unit Doctor. 54 (6): 1971–6. PMID 8900357.
- ^ Karlsson U, Bjöersdorff A, Massung RF, Christensson B (2001). "Human granulocytic ehrlichiosis--a clinical example in Scandinavia". Scandinavian Journal of Infectious Diseases. 33 (1): 73–4. doi:ten.1080/003655401750064130. PMID 11234985. S2CID 218880245.
- ^ a b c d e f U.S. Food and Drug Administration. fourteen December 2012. Doxycycline, ANDA no. 065055 Label. Archived 19 April 2014 at the Wayback Machine
- ^ a b c d e U.Due south. Nutrient and Drug Administration. 16 July 2008.Doxycycline, ANDA no. 065454 Label Archived nineteen October 2013 at the Wayback Machine
- ^ Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. (March 2013). "Diagnosis and direction of Q fever--Us, 2013: recommendations from CDC and the Q Fever Working Grouping". MMWR. Recommendations and Reports. 62 (RR-03): 1–thirty. PMID 23535757. Archived from the original on 19 April 2014.
- ^ Okada T, Morozumi M, Tajima T, Hasegawa G, Sakata H, Ohnari S, et al. (Dec 2012). "Rapid effectiveness of minocycline or doxycycline confronting macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children". Clinical Infectious Diseases. 55 (12): 1642–9. doi:10.1093/cid/cis784. PMID 22972867.
- ^ "Lyme illness. Treatment". 21 Dec 2018. Archived from the original on 10 June 2016.
- ^ Dreno B, Thiboutot D, Gollnick H, Bettoli V, Kang S, Leyden JJ, et al. (2014). "Antibiotic stewardship in dermatology: limiting antibiotic utilise in acne". European Periodical of Dermatology. 24 (iii): 330–iv. doi:x.1684/ejd.2014.2309. PMID 24721547. S2CID 28700961.
- ^ William Cameron (2012). "Comparing of doxycycline–streptomycin, doxycycline–rifampin, and ofloxacin–rifampin in the handling of brucellosis: a randomized clinical trial". International Journal of Infectious Diseases. 16 (4): e247–e251. doi:10.1016/j.ijid.2011.12.003. PMID 22296864. Retrieved 23 August 2014.
- ^ "Doryx- doxycycline hyclate tablet, delayed release". DailyMed. 23 Oct 2020. Retrieved 5 March 2022.
- ^ "Malaria - Chapter three - 2018 Yellow Volume | Travelers' Health | CDC". CDC . Retrieved 4 December 2018.
- ^ Dahl EL, Stupor JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ (September 2006). "Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum". Antimicrobial Agents and Chemotherapy. fifty (9): 3124–31. doi:10.1128/AAC.00394-06. PMC1563505. PMID 16940111.
- ^ Guidelines for the handling of malaria. Geneva: Globe Health Arrangement. 2015. p. 246. ISBN978-92-4-154912-seven.
- ^ Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, et al. (Nov 2003). "Doxycycline as a novel strategy confronting bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production". Medical Microbiology and Immunology. 192 (4): 211–half dozen. doi:x.1007/s00430-002-0174-half-dozen. PMID 12684759. S2CID 23349595.
- ^ Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand South, Hoerauf A (2005). "Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial". Lancet. 365 (9477): 2116–21. doi:x.1016/S0140-6736(05)66591-9. PMID 15964448. S2CID 21382828.
- ^ "Doxycycline hyclate Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). toku-e.com . Retrieved sixteen April 2017.
- ^ Kaufman, John A.; Lee, Michael J. (22 June 2013). Vascular and interventional radiology (2nd ed.). Philadelphia, PA. ISBN978-0-323-07672-2. OCLC 853455295.
- ^ Sgolastra F, Petrucci A, Gatto R, Giannoni M, Monaco A (November 2011). "Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-assay". Journal of Periodontology. 82 (xi): 1570–81. doi:10.1902/jop.2011.110026. PMID 21417590.
- ^ Chung AM, Reed MD, Blumer JL (2002). "Antibiotics and chest-feeding: a disquisitional review of the literature". Paediatric Drugs. 4 (12): 817–37. doi:x.2165/00128072-200204120-00006. PMID 12431134. S2CID 8595370.
- ^ Mylonas I (Jan 2011). "Antibody chemotherapy during pregnancy and lactation period: aspects for consideration". Archives of Gynecology and Obstetrics. 283 (1): vii–18. doi:10.1007/s00404-010-1646-3. PMID 20814687. S2CID 25492353.
- ^ "Bioterrorism and Drug Preparedness - Doxycycline Use past Pregnant and Lactating Women". FDA. 3 November 2018. Retrieved 9 December 2018.
- ^ Gaillard T, Briolant Due south, Madamet M, Pradines B (Apr 2017). "The end of a dogma: the safety of doxycycline utilize in young children for malaria treatment". Malaria Journal. xvi (1): 148. doi:x.1186/s12936-017-1797-ix. PMC5390373. PMID 28407772.
- ^ a b c d Haberfeld H, ed. (2020). Austria-Codex (in High german). Vienna: Österreichischer Apothekerverlag. Doxycyclin Genericon 200 mg lösliche Tabletten.
- ^ a b Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2015). Top 100 drugs : clinical pharmacology and practical prescribing. pp. 200–201. ISBN978-0-7020-5516-4.
- ^ a b Riond JL, Riviere JE (October 1988). "Pharmacology and toxicology of doxycycline". Veterinary and Human Toxicology. xxx (5): 431–43. PMID 3055652.
- ^ Affolter Thou, Samowitz W, Boynton Thousand, Kelly ED (Baronial 2017). "Doxycycline-induced gastrointestinal injury". Human Pathology. 66: 212–215. doi:10.1016/j.humpath.2017.02.011. PMID 28286288.
- ^ Hung YP, Lee JC, Lin HJ, Liu HC, Wu YH, Tsai PJ, Ko WC (June 2015). "Doxycycline and Tigecycline: Two Friendly Drugs with a Depression Association with Clostridium Difficile Infection". Antibiotics. iv (two): 216–29. doi:ten.3390/antibiotics4020216. PMC4790331. PMID 27025622.
- ^ Tan KR, Magill AJ, Parise ME, Arguin PM (April 2011). "Doxycycline for malaria chemoprophylaxis and treatment: written report from the CDC expert meeting on malaria chemoprophylaxis". The American Periodical of Tropical Medicine and Hygiene. 84 (four): 517–31. doi:10.4269/ajtmh.2011.10-0285. PMC3062442. PMID 21460003. [ verification needed ]
- ^ Dréno B, Bettoli 5, Ochsendorf F, Layton A, Mobacken H, Degreef H (November–December 2004). "European recommendations on the utilise of oral antibiotics for acne". European Journal of Dermatology. 14 (half-dozen): 391–9. PMID 15564203. Archived from the original on 29 May 2007. Retrieved 31 Jan 2008. [ verification needed ]
- ^ Lee TW, Russell L, Deng 1000, Gibson PR (Baronial 2013). "Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel affliction in Australians deployed abroad". Internal Medicine Journal. 43 (8): 919–26. doi:10.1111/imj.12179. PMID 23656210. S2CID 9418654.
- ^ Margolis DJ, Fanelli Grand, Hoffstad O, Lewis JD (December 2010). "Potential clan between the oral tetracycline course of antimicrobials used to treat acne and inflammatory bowel disease". The American Periodical of Gastroenterology. 105 (12): 2610–6. doi:10.1038/ajg.2010.303. PMID 20700115. S2CID 20085592.
- ^ PubMed Health (1 July 2016). "Doxycycline (Past mouth)". U.Due south. National Library of Medicine. Archived from the original on 11 November 2013. Retrieved xvi July 2016.
- ^ Kshirsagar N A, Ankalesaria P S. Outcome of food on doxycycline absorption. J Postgrad Med (serial online) 1987 (cited 2016 Jul 16);33:117. Bachelor from: http://www.jpgmonline.com/text.asp?1987/33/3/117/5279 Archived xviii Baronial 2016 at the Wayback Machine
- ^ Archer JS, Archer DF (June 2002). "Oral contraceptive efficacy and antibiotic interaction: a myth debunked". Journal of the American Academy of Dermatology. 46 (half-dozen): 917–23. doi:10.1067/mjd.2002.120448. PMID 12063491.
- ^ Dréno B, Bettoli 5, Ochsendorf F, Layton A, Mobacken H, Degreef H (November–December 2004). "European recommendations on the use of oral antibiotics for acne" (PDF). European Journal of Dermatology. 14 (6): 391–9. PMID 15564203. [ permanent dead link ]
- ^ DeRossi SS, Hersh EV (October 2002). "Antibiotics and oral contraceptives". Dental Clinics of Due north America. 46 (4): 653–64. CiteSeerX10.1.one.620.9933. doi:x.1016/S0011-8532(02)00017-4. PMID 12436822.
- ^ Flower, R.; Rang, H. P.; Dale, Chiliad. M.; Ritter, J. Grand.; Henderson, G. (2012). Rang & Dale's Pharmacology. Edinburgh: Churchill Livingstone. ISBN978-0-7020-3471-8.
- ^ a b Maaland MG, Papich MG, Turnidge J, Guardabassi L (November 2013). "Pharmacodynamics of doxycycline and tetracycline against Staphylococcus pseudintermedius: proposal of canine-specific breakpoints for doxycycline". Journal of Clinical Microbiology. 51 (eleven): 3547–54. doi:ten.1128/JCM.01498-13. PMC3889732. PMID 23966509.
- ^ Agwuh KN, MacGowan A (August 2006). "Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines". The Periodical of Antimicrobial Chemotherapy. 58 (2): 256–65. doi:x.1093/jac/dkl224. PMID 16816396.
- ^ "Doxycycline". world wide web.drugbank.ca . Retrieved 23 January 2019.
- ^ a b c d eastward Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). Vol. four (24 ed.). Eschborn, Deutschland: Govi Pharmazeutischer Verlag. Doxycyclin. ISBN978-3-7741-9846-three.
- ^ a b Doxycycline Professional Drug Facts. Accessed five Baronial 2020.
- ^ Haberfeld H, ed. (2020). Austria-Codex (in High german). Vienna: Österreichischer Apothekerverlag. Minostad 50 mg-Kapseln.
- ^ "Principles and methods for the cess of nephrotoxicity associated with exposure to chemicals" Archived x May 2011 at the Wayback Machine. Environmental health criteria: 119. Earth Health Organization (WHO). ISBN 92-iv-157119-5. ISSN 0250-863X. 1991.
- ^ a b Foye'southward Principles of Medicinal Chemistry; David A. Williams; William O. Foye, Thomas Fifty. Lemke
- ^ a b Goodman & Gilman's The Pharmacological Basis of Therapeutics, 12ed, Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann
- ^ Pfizer, Inc. v. International Rectifier Corp., 545 F. Supp. 486 (C.D. Cal. 1980) Archived 24 February 2015 at the Wayback Machine
- ^ a b The Associated Press, vi July 1983 "Pfizer to Get Rachelle Units" Archived 6 March 2016 at the Wayback Motorcar The New York Times.
- ^ CDC Wellness Warning Network 12 June 2013 Nationwide Shortage of Doxycycline: Resources for Providers and Recommendations for Patient Care Archived 15 February 2015 at the Wayback Machine
- ^ American Society of Health-Organisation Pharmacists. 12 December 2014 Doxycycline Capsules and Tablets Archived 1 Jan 2015 at the Wayback Car
- ^ American Society of Health-System Pharmacists. 12 November 2014 Doxycycline Hyclate Injection Archived 1 January 2015 at the Wayback Machine
- ^ Consumer Reports News: 4 February 2013 FDA reports shortage of doxycycline antibiotic. What are your options? Archived 1 January 2015 at the Wayback Machine
- ^ Sudden increase in cost of common drug concerns many Archived 31 December 2014 at the Wayback Machine, WSMV-TV, 12 March 2013 (updated 26 March 2013).
- ^ Rosenthal, Elisabeth, Officials Question the Ascent Costs of Generic Drugs Archived 23 February 2017 at the Wayback Machine, The New York Times, seven Oct 2014.
- ^ Eric Palmer for FiercePharmaManufacturing. 13 March 2014 Hikma hits the jackpot with doxycycline shortage Archived 1 January 2015 at the Wayback Automobile
- ^ "Costco Drug Information". Archived from the original on iv March 2016. Retrieved 31 July 2016.
- ^ "Doxycycline Hyclate Prices and Doxycycline Hyclate Coupons". GoodRx. Archived from the original on 28 July 2016. Retrieved 31 July 2016.
- ^ drugs.com Drugs.com international availability for doxycycline Archived 16 May 2015 at the Wayback Car Page accessed 29 April 2015
- ^ Leung Eastward, Landa Thou (September 2013). "Update on current and future novel therapies for dry out age-related macular degeneration". Expert Review of Clinical Pharmacology. half-dozen (5): 565–79. doi:10.1586/17512433.2013.829645. PMID 23971874. S2CID 26680094.
- ^ Greenwald RA (December 2011). "The road forward: the scientific basis for tetracycline treatment of arthritic disorders". Pharmacological Enquiry. 64 (half dozen): 610–iii. doi:10.1016/j.phrs.2011.06.010. PMID 21723947.
- ^ Moullan Due north, Mouchiroud L, Wang 10, Ryu D, Williams EG, Mottis A, et al. (March 2015). "Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research". Prison cell Reports. 10 (x): 1681–1691. doi:10.1016/j.celrep.2015.02.034. PMC4565776. PMID 25772356.
- ^ Chatzispyrou IA, Held NM, Mouchiroud 50, Auwerx J, Houtkooper RH (November 2015). "Tetracycline antibiotics impair mitochondrial role and its experimental apply confounds research". Cancer Research. 75 (21): 4446–nine. doi:x.1158/0008-5472.Tin-15-1626. PMC4631686. PMID 26475870.
- ^ a b Gossen Thousand, Freundlieb Southward, Bender G, Müller G, Hillen W, Bujard H (June 1995). "Transcriptional activation past tetracyclines in mammalian cells". Science. 268 (5218): 1766–9. Bibcode:1995Sci...268.1766G. doi:x.1126/scientific discipline.7792603. PMID 7792603.
- ^ Dursun D, Kim MC, Solomon A, Pflugfelder SC (July 2001). "Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-nine, doxycycline and corticosteroids". American Journal of Ophthalmology. 132 (1): viii–13. doi:ten.1016/S0002-9394(01)00913-8. PMID 11438047.
External links [edit]
- "Doxycycline". Drug Information Portal. U.S. National Library of Medicine.
Source: https://en.wikipedia.org/wiki/Doxycycline
Posted by: woodardaffeekly.blogspot.com

![{\displaystyle [\alpha ]_{D}^{25}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fc055b4d62c591651f8a4adbc6f6b2e9e71ce021)
0 Response to "What Is The Chemical Makeup Of Doxycycline Hyclate"
Post a Comment